Newborn Hearing Screening
The scope of this page is hearing screening for infants aged birth to 6 months. Newborn hearing screening is one part of a comprehensive Early Hearing Detection and Intervention (EHDI) program of service.
See the Screening section of the Hearing Loss (Newborn) Evidence Map for pertinent scientific evidence, expert opinion, and client/caregiver perspective.
Hearing-related terminology may vary depending upon context and a range of factors. See the ASHA resource on hearing-related topics: terminology guidance for more information.
Newborn hearing screening is the standard of care in hospitals nationwide. The primary purpose of newborn hearing screening is to identify newborns who are likely to have hearing loss and who require further evaluation. A secondary objective is to identify newborns with medical conditions that can cause late-onset hearing loss and establish a plan for continued monitoring of their hearing status (Joint Committee on Infant Hearing [JCIH], 2019). Benchmarks recommended by EHDI programs include hearing screening completion by 1 month of age, audiological diagnosis of any hearing loss by 3 months of age, hearing aid selection and fitting within 1 month of confirmation of hearing loss if parents/guardians choose that option, and entry into early intervention services by 6 months of age. States meeting the 1–3–6-month benchmarks should strive to meet a 1–2–3-month timeline, including hearing screening by 1 month of age, audiological diagnosis of any hearing loss by 2 months of age, and enrollment in early intervention by 3 months of age (JCIH, 2019). See the JCIH Year 2019 Position Statement and the ASHA Practice Portal page on Early Intervention for more information.
In 2020, 97.2% of babies born in the United States had their hearing screened before 1 month of age (Centers for Disease Control and Prevention, 2023), and 6,291 infants were diagnosed with permanent hearing loss.
Screening programs target permanent childhood hearing loss irrespective of type. However, some protocols are more effective at identifying types and degrees of hearing loss within different populations (i.e., well-baby nursery or neonatal intensive care unit).
Passing a screening does not mean that a child has typical hearing across the frequency range. Minimal and frequency-specific hearing losses are not targeted by newborn hearing screening programs. Current screening technology is effective in identifying hearing thresholds of 35–40 dB HL and greater (Norton et al., 2000) and may fail to identify mildly elevated hearing thresholds (JCIH, 2019). Therefore, newborns with mild hearing loss may pass a hearing screening. Because mild hearing loss has the potential to interfere with the speech, language, and psychoeducational development of children (Yoshinaga-Itano et al., 2008), monitoring of hearing, speech, and language milestones throughout childhood is essential.
Roles and Responsibilities
Roles and Responsibilities of Audiologists
Audiologists, by virtue of academic degree, clinical training, and license to practice, are qualified to provide guidance, development, implementation, and oversight of newborn hearing screening programs. Professional roles and activities in audiology include clinical and educational services (e.g., screening); prevention and advocacy; and education, administration, and research. See ASHA’s Scope of Practice in Audiology (ASHA, 2018).
The following roles and responsibilities are appropriate for audiologists:
- Provide management and oversight to trained professionals performing screenings.
- Train and monitor (on a continual basis) screening personnel competencies.
- Select screening protocols for both the neonatal intensive care unit (NICU) and the well-baby nursery.
- Select screening technology and equipment.
- Perform hearing screenings.
- Select or develop educational materials.
- Monitor key indicators (e.g., refer rates, miss rates) and provide quality assurance.
- Develop and implement written policies and procedures on
- infection control;
- screening techniques and process;
- documentation of screening results per medical facility protocol (e.g., medical records, electronic health record, birth certificate, discharge summary); and
- calibration and upgrade of equipment.
- Communicate screening results to families, primary care physicians, and diagnostic audiology centers, as indicated.
- Report results to the state-specific early hearing detection and intervention (EHDI) system, including results of all follow-up assessments and appointments.
- Provide counseling and education.
- Refer infants for audiologic and medical services, as indicated.
- Advocate for the communication needs of all individuals, including advocating for the rights to and funding of services for those with hearing loss.
As indicated in the Code of Ethics (ASHA, 2023), audiologists who work in this capacity should be specifically educated and appropriately trained to do so. Audiologist oversight is recommended for all state/territory hearing screening programs, both at the systems level and at the individual program level (Joint Committee on Infant Hearing [JCIH], 2019).
Roles and Responsibilities of Speech-Language Pathologists
Speech-language pathologists (SLPs) may be called upon to perform newborn hearing screenings. SLPs play a role in the hearing screening process and in the referral of individuals suspected of having hearing loss to an audiologist. Professional roles and activities in speech-language pathology include clinical and educational services (e.g., screening); prevention and advocacy; and education, administration, and research. See ASHA’s Scope of Practice in Speech-Language Pathology (ASHA, 2016).
The following roles are appropriate for SLPs within newborn hearing screening programs:
- Perform newborn hearing screenings using automated equipment in the hospital and as part of outpatient screening programs with appropriate training and oversight from the managing audiologist.
- Communicate screening results to families, including recommendations for follow-up consistent with JCIH recommendations.
- Document and share screening results with state public health agencies, primary care physicians, and diagnostic audiology centers as required by the state EHDI program.
- Collaborate with audiologists, physicians, nurses, and/or other professionals to ensure timely follow-up.
- Refer infants for comprehensive audiologic, medical, and/or other professional services, as indicated.
- Advocate for the communication needs of all individuals, including advocating for the rights to and funding of services for those with hearing loss.
As indicated in the Code of Ethics (ASHA, 2023), SLPs who work in this capacity should be specifically educated and appropriately trained to do so.
General Considerations
Universal newborn hearing screening programs typically include the following components:
- written educational materials for parents/guardians
- a process for obtaining parent/guardian consent in accordance with state and federal guidelines
- comprehensive, competency-based screener training
- infection control procedures
- hearing screening protocols using objective physiological test(s)
- a process for communicating screening results (e.g., parents/guardians, primary care physicians, state EHDI programs)
- a follow-up system for infants who do not pass inpatient hearing screening, who are at risk for late-onset and progressive hearing loss, or who are missed by the inpatient screening program—to include repeat screening or referral for appropriate evaluation and early intervention
- means of connecting families with support organizations that focus on the needs of newly identified infants, children who are deaf and hard of hearing, and their families
- documentation and data systems to track screening and follow-up
- access to interpreters who use languages represented by the population served
- a quality assurance process to evaluate the effectiveness of newborn hearing screening (JCIH, 2019)
A state policy of performing screening without obtaining parent/guardian permission, or at least informing parents/guardians about screening, may violate constitutional protections of the parental/guardianship role (Berge, 1992; Clayton, 1992; Fleischman et al., 1994). Although many hospitals have global consents for all newborn hearing screening procedures, screeners and personnel should be aware of hospital and state regulations regarding parent/guardian refusal.
See the ASHA state-by-state resource for more information.
Screener Roles and Characteristics
Various personnel may perform newborn hearing screenings. A screener may be an audiologist, an SLP, a nurse, a paraprofessional, or a trained volunteer. All screeners are trained in, and competent to use, the technology and protocol specific to the screening program. It is important for the screener to have familiarity with JCIH guidelines, EHDI processes, and hospital/clinic protocols, including standard precautions and patient confidentiality standards.
Timing of Screening
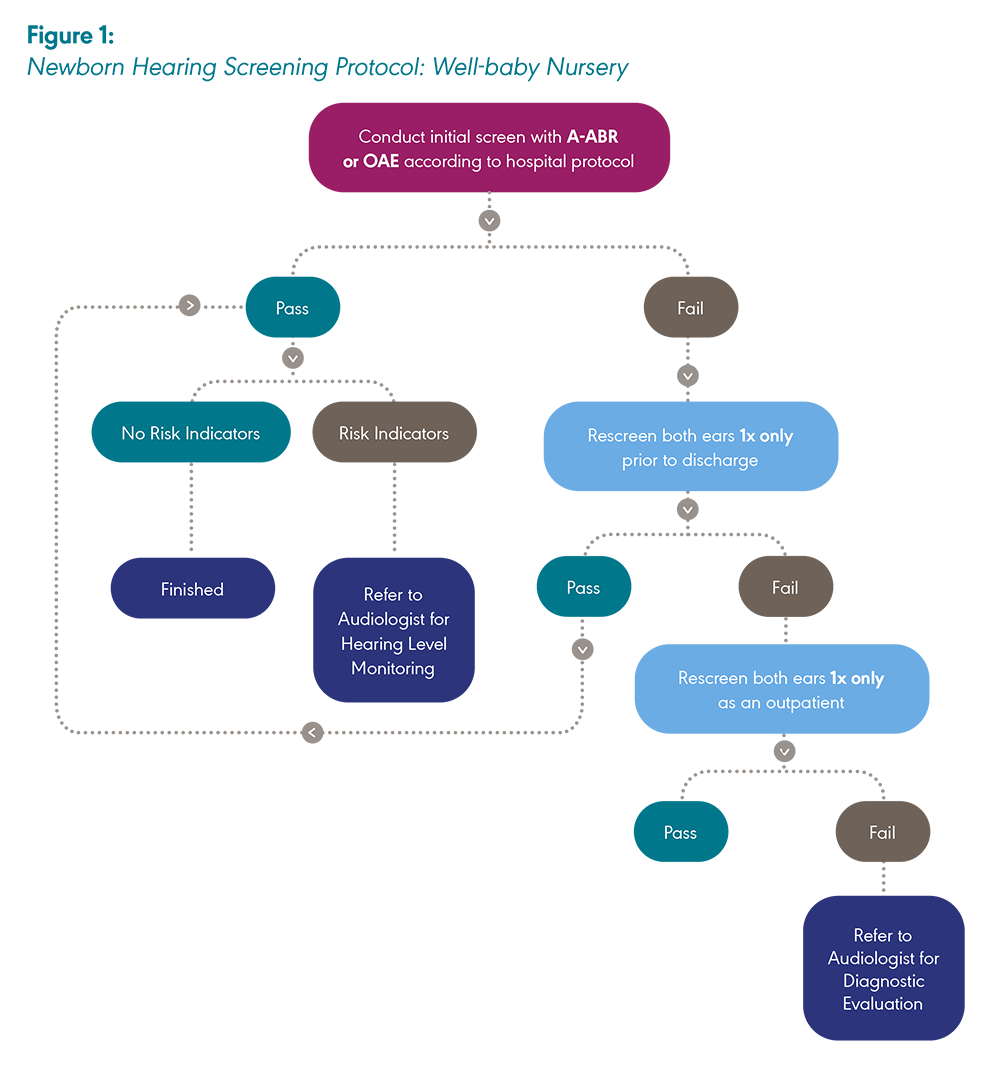
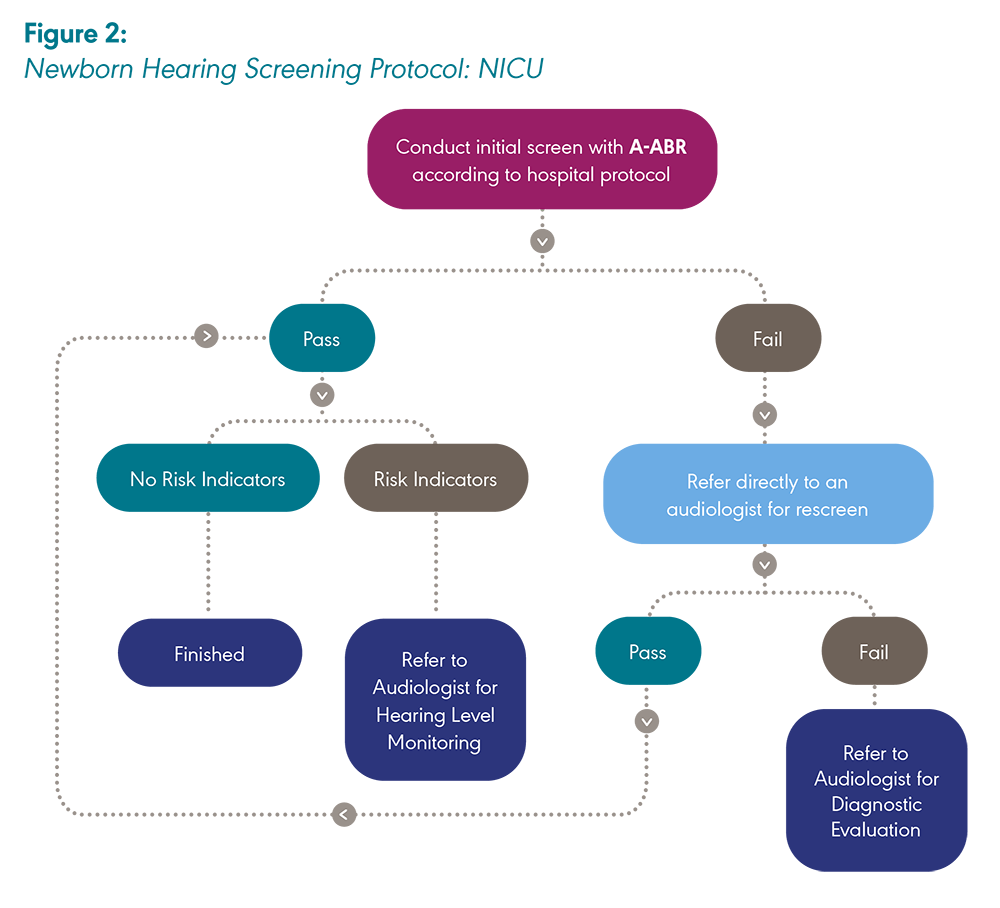
Newborns cared for in the well-baby nursery are screened as close to hospital discharge as possible while, at the same time, allowing sufficient time for a single repeat screen to be performed if the infant does not pass the initial screen. A second screen should not be performed immediately following the first screen but should occur several hours later (JCIH, 2019). Newborn hearing screenings should occur before 1 month of age at the latest. Newborns in the NICU are screened when they are ready for discharge and/or when they are medically stable.
Newborns who have initially passed a hearing screening are rescreened if readmitted to the hospital in the first month of life or if risk factors for hearing loss develop during the infant’s hospital stay following the initial screening. State laws and hospital protocols may vary regarding which hospital is responsible for screening newborns who are transferred from one hospital to another.
Testing Environment
Screening can be done in a nursery or another quiet room, with the infant resting quietly or sleeping. A sound booth is not needed. The preferred method is to test the newborn while they are resting quietly in their bassinet. If needed, the newborn can be held. “A high-quality screen implies that the infant is sleeping or resting quietly without movement throughout the screening period, and that patency of the ear canal is assured to the extent possible prior to the screen” (JCIH, 2019, p. 8).
Pass/Refer Indications
A newborn must pass the screening in both ears during one session for the screening to be considered a “pass.” Otherwise, the newborn will be referred for rescreening. If the newborn does not pass in one ear, both ears must be rescreened. If the newborn passes the screening or the rescreening and has no risk factors for late-onset or progressive hearing loss, then the screening is complete. If the newborn passes the screening or the rescreening and has risk factors for late-onset or progressive hearing loss, then it will be particularly important to monitor the newborn’s hearing during early childhood (Harlor & Bower, 2009; JCIH, 2007). In some settings and/or instances, the terms “fail” or “did not pass” may be used interchangeably with the term “refer.”
Care should be taken not to screen newborns more times than recommended in the protocol. The probability of an erroneous “pass” outcome (i.e., of infants with hearing loss passing the screening) increases with every screen.
Standard Universal Precautions
All procedures must ensure the safety of the patient and clinician and adhere to universal health precautions (e.g., prevention of bodily injury and transmission of infectious disease). Decontamination, cleaning, disinfection, and sterilization of multiple-use equipment before reuse must be carried out according to facility-specific infection control policies and procedures and according to the manufacturer’s instructions (Siegel et al., 2007). See ASHA’s infection control resources for audiologists and speech-language pathologists for more information.
Technology
Automated auditory brainstem response (A-ABR) and otoacoustic emissions (OAEs) are appropriate physiologic measures for screening the newborn population. Both are noninvasive and available in automated versions that are easily utilized by trained staff.
Both A-ABR and OAE technologies will miss delayed-onset hearing loss, mild hearing loss, or hearing loss that is present only at isolated frequencies. Both A-ABR and OAE responses are affected by outer or middle ear dysfunction. When a transient middle ear condition is present, both technologies will likely result in the newborn not passing the screening. Both OAE and A-ABR screening reflect physiologic processes within the auditory system and identify hearing loss most accurately from 2000 Hz to 4000 Hz.
Automated technologies (i.e., those that determine pass/refer) do not require interpretation. Automated screening equipment often has test parameters set by the manufacturer. Therefore, different equipment may yield different screening results.
Even if diagnostic (i.e., nonautomated) technology is used with an audiologist interpreting the results, procedures in the nursery are limited to screening (i.e., pass/refer). All equipment used should be calibrated and maintained according to the manufacturer’s specifications.
The factors that influence the selection of screening technology include
- the population to be screened (i.e., well-baby nursery vs. NICU);
- the average length of stay in facility;
- the size of the screening population;
- the time required of the screener; and
- the cost (e.g., equipment purchases and calibration, disposable equipment).
Automated Auditory Brainstem Response (A-ABR)
A-ABR activity is a direct measurement of the neural response to sound that is generated along the auditory system from the level of the cochlea and through the vestibulocochlear nerve (CN VIII) and pontine level of the brainstem and that correlates with behavioral hearing measures in the mid- to high-frequency region. The A-ABR is recorded using surface electrodes that are attached to the infant’s head. Click stimuli are presented through a probe or earphones (i.e., insert or muff-style) worn on both ears. A-ABR measurements are sensitive to neural auditory disorders (e.g., auditory neuropathy). Auditory neuropathy is a neural hearing loss that leaves cochlear (outer hair cell) function intact. It is more prevalent in the NICU population than in the well-baby nursery population (D’Agostino & Austin, 2004). A-ABR screening is less sensitive to outer ear debris than OAE screening, resulting in lower referral rates.
Stimuli
Most automated equipment presents click stimuli at 35 dB nHL at a rate of 30–37 clicks per second. If the equipment allows a choice of stimulus levels, the screening program audiologist can adjust to lower click levels.
Response Criteria
For A-ABR, manufacturers use their own proprietary stopping rule, based on a template comparison or statistical algorithms. Most instrumentation does not allow operators to change the stopping rule criteria.
Procedure
Prior to initiating the screening, it is important to ensure that the equipment is working properly and that a quiet screening environment is available. The infant should be asleep or resting quietly for the test and positioned to reduce muscle artifact. The screener visually inspects the outer part of the ear canal to ensure that the canal is clear of debris and then places the transducer. Both ears are screened during each session.
Otoacoustic Emissions (OAE)
OAEs—either transient-evoked OAEs (TEOAEs) or distortion product OAEs (DPOAEs)—are measured using a sensitive probe microphone inserted into the infant’s ear canal. OAEs are a direct measure of outer hair cell and cochlear function in response to acoustic stimulation. They yield an indirect estimate of peripheral hearing sensitivity. OAEs are not sensitive to disorders central to the outer hair cells, such as auditory neuropathy. OAEs will be absent when there is outer or middle ear dysfunction or debris/blockage in the ear canal.
Stimuli
TEOAEs use a high-level click, approximately 80 dB pSPL, and a subtraction (sometimes referred to as nonlinear) paradigm to reduce stimulus artifact. DPOAEs use mid-level stimuli (f1 primary = 65 dB SPL and f2 primary = 55 dB SPL).
Response Criteria
Many manufacturers program response criteria into the OAE unit. At least three test frequencies—2000 Hz, 3000 Hz, and 4000 Hz—are evaluated during the screening. Usually, signal-to-noise ratios (SNRs) of at least 6 dB are used. Some manufacturers will set their own SNRs. DPOAE SNRs vary depending on the calculation used (i.e., mean SNR or mean +1 or +2 SDs of noise). A minimum absolute DPOAE level of 5 dB SPL is imposed.
Typically, a minimum of 50 averages is collected before testing is terminated when using TEOAEs. DPOAEs are often terminated based on SNR. Rather than a minimum number of averages, manufacturers choose a minimum length of averaging time.
Procedure
Prior to initiating the screening, it is important to ensure that the equipment is working properly and that a quiet screening environment is available. The infant should be asleep or resting quietly. A snug probe fit is essential for valid and reliable recordings. Ears should be screened one at a time, with the infant placed on their side and the ear being screened facing up. The screener visually inspects the outer part of the ear canal to ensure that the canal is patent and clear of debris. Prior to insertion of the probe, a gentle massage of the area below the tragus helps to open a collapsed canal or dislodge debris that may be blocking the canal.
In OAE screenings, the stimulus level is calibrated in each ear according to the manufacturer’s specifications. After stimulus-level requirements have been met, OAEs are collected to meet stopping criteria. If OAEs do not appear to be present, the probe is removed and inspected to determine if the probe is blocked with cerumen or vernix. A blocked probe can be cleaned and reinserted, and the screening then repeated. Both ears are screened during each session.
Protocols
Newborn hearing screening protocols can be broadly classified into two categories: one used in the well-baby nursery setting and one used in the NICU.
In the well-baby protocol, regardless of the equipment used (i.e., A-ABR or OAE), the screening process is the same. This includes one inpatient screening with one inpatient rescreen if needed. Both ears are screened during each session, even if one ear passes the initial screening. An outpatient rescreen, if required, is comprised of a single valid rescreen of both ears during one session (JCIH, 2019).
A-ABR screening is recommended by the JCIH (2019) for newborns cared for in the NICU.
The choice of protocol for a newborn hearing screening program is based on the specific needs of the population to be screened and the requirements of the state or hospital program.
Note: A-ABR = Automated auditory brainstem response; OAE = Otoacoustic emission. Created by ASHA with information adapted from “Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs,” by The Joint Committee on Infant Hearing, 2019, The Journal of Early Hearing Detection and Intervention, 4(2), 1–44.
Note: A-ABR = Automated auditory brainstem response; OAE = Otoacoustic emission. Created by ASHA with information adapted from “Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs,” by The Joint Committee on Infant Hearing, 2019, The Journal of Early Hearing Detection and Intervention, 4(2), 1–44.
For infants who are born outside of a hospital setting (e.g., home birth or birthing center), state EHDI program guidelines inform the process for obtaining a newborn hearing screening. “A pathway for initial screening for infants who, for a variety of reasons, miss the initial screening . . . should be developed and followed such that no infant will be inadvertently missed” (JCIH, 2019, p. 12).
Protocol Considerations
Consider the following factors when using an A-ABR-only screening approach:
- A-ABR can be used in both the NICU and the well-baby nursery. The rationale for an “A-ABR-only” protocol is that both neural and cochlear hearing losses will be detected using one type of technology.
- More of the auditory system is accessed with A-ABR screening than with OAEs, allowing for detection of neurologic involvement.
- A-ABR screening results are less susceptible to false positives due to ear canal debris than OAE screenings (Vohr et al., 2001).
- An A-ABR screening can be less cost effective than an OAE screening because of the higher cost of disposables (i.e., electrodes and disposable earphones) and increased personnel time (Berg et al., 2011). This cost differential may be balanced with a lower refer rate, resulting in reportedly lower or similar overall program costs (Vohr et al., 2001).
- A-ABR test time can be longer than OAE test time because of electrode application (Berg et al., 2011).
- A-ABR will result in false positives when used with babies who have immature neurological systems that affect the A-ABR waveform (Turchetta et al., 2012).
Consider the following factors when using an OAE-only screening approach:
- OAE disposable supplies are often less expensive than those used with A-ABR.
- OAE test time has been reported to be shorter than A-ABR test time (Berg et al., 2011).
- OAEs do not detect neural hearing losses.
- OAEs have a higher inpatient refer rate as compared to A-ABR. Outpatient rescreening is advised for newborns who do not pass inpatient screening before referral to an audiologist for diagnostic testing.
- OAEs may be reduced or absent due to outer ear debris and/or middle ear fluid common in the newborn population, resulting in higher refer rates. That is, OAEs have a higher false positive rate than A-ABR.
Consider the following factors when using a two-stage screening approach (OAE screening followed by A-ABR screening for infants who do not pass the OAE screening):
- The number of referrals for diagnostic testing will be lower in a two-stage protocol than in an OAE-only protocol.
- Two types of disposables will be required, and two types of screening technology may be needed, although some manufacturers offer both tests on one piece of equipment.
- Personnel must be trained in two procedures.
- Because tests detect different degrees of hearing loss, a baby may not pass the OAE screening but pass the A-ABR screening (Johnson et al., 2005). As a result, this protocol may miss some children with mild hearing loss.
- A two-stage protocol may miss auditory neuropathy because infants who pass an OAE screening will not be referred for an A-ABR screening.
Detection of Late-Onset Hearing Loss
The American Academy of Pediatrics (AAP) recommends that every newborn have a medical home to provide coordinated and comprehensive primary care that is both accessible and family-centered. The medical home plays a key role in supporting EHDI systems and detecting late-onset hearing loss (Mehl & Esquivel, 2016). Although initial newborn hearing screenings are not typically conducted in the medical home, the JCIH recommends that the pediatric primary care provider regularly monitor all infants and children for hearing loss and communication development in the medical home (JCIH, 2007). This allows for the identification of children with missed newborn, late-onset, or progressive hearing loss, regardless of the presence or absence of high-risk indicators at birth. The onset of hearing loss can occur at any time in a child’s life. Developmental milestones, hearing skills, and speech and language milestones should be monitored in all children, consistent with the AAP’s Recommendations for Preventive Pediatric Health Care [PDF]. See the ASHA Practice Portal page on Childhood Hearing Screening and the JCIH Year 2019 Position Statement for more information on this topic.
Documentation and Dissemination
Newborn hearing screening documentation requirements are based on hospital and state mandates or protocols and can include the recording of screening results into the medical record, electronic health record, birth certificate, discharge summary, and/or state EHDI data system. In addition, screening results are provided to the newborn’s family and physician. Patient records must be in full compliance with the Health Insurance Portability and Accountability Act of 1996.
Documentation of newborn hearing screening typically includes the following information:
- child’s name and date of birth
- parent/guardian contact information
- date and time of all screenings
- type of screening (OAE, A-ABR, or both)
- outcome of the screening for each ear
- any known risk factors
- recommendations for next steps
- follow-up information, if applicable (e.g., appointment date/time/place, phone number of the follow-up facility)
- hospital transfer information, if applicable
- signature and credentials of personnel conducting the screening
Collecting parent/guardian and primary care physician contact information is critical in the effort to prevent loss to follow-up, especially for those newborns who do not pass the screening. To track newborn screenings effectively, states stipulate which information will be reported to the appropriate government agency (e.g., state EHDI program). See the ASHA state-by-state resource for a summary of requirements.
Counseling and Education
Providing counseling and education is an important part of the newborn hearing screening process. See the ASHA Practice Portal page on Counseling in Audiology and Speech-Language Pathology for more information.
Parent/Guardian Education
Educational materials can be included in patient education packets, as part of hospital prenatal education programs, and in public health clinic outreach programs. Education regarding newborn hearing screening begins before an infant is screened. Materials should be health literate, culturally appropriate, and available in the language used by the family. Information should be conveyed orally in the language used by the parents/guardians.
Educational materials typically include information on
- the importance of early hearing detection and intervention,
- the screening process,
- screening safety and noninvasive methods,
- the meaning of “pass” and “refer” results,
- the importance of follow-up after screening,
- the steps involved in declining screening,
- risk factors for late-onset hearing loss, and
- developmental milestones for typically developing children.
Communicating Screening Results
Where possible, audiologists are the professionals who communicate hearing screening results to the parents/guardians. When an audiologist is not available to convey results, other qualified individuals (e.g., SLPs, nurses, technicians, physicians) will provide and explain screening outcomes. See the ASHA resource on health literacy for more information.
When communicating screening results, consider the following principles:
- Present information in a confidential and family-centered manner.
- Use clear and concise wording that avoids technical jargon.
- Provide information in the mode of communication and language used by the family.
- Present comprehensive findings free from speculation and/or opinion.
- Emphasize appropriate follow-up.
- Give parents/guardians the opportunity to ask questions.
If follow-up is needed, provide the following information to the parents/guardians:
- explicit recommendations on how to secure follow-up testing
- contact information for an audiologist whom they can contact directly with questions in the interim
- available local, state, and national resources that they can use to obtain information about subsequent stages of the EHDI process
The importance of ongoing surveillance and periodic monitoring is emphasized to families of newborns who pass the hearing screening as well.
Loss to Follow-Up and Loss to Documentation
In general, lost to follow-up (LTF) is a designation for an infant who does not complete the recommended diagnostic or intervention process following the hearing screening. Lost to documentation (LTD) designates those infants who did not pass their hearing screening and have had no diagnostic or intervention status reported to the EHDI program.
There has been a slow but steady decline in the U.S. LTF/LTD rates. In 2020, 29.9% of the infants who did not pass their final newborn hearing screening did not complete follow-up and were categorized as LTF/LTD (Centers for Disease Control and Prevention [CDC], 2023).
Populations at particular risk for LTF include infants born in home births, babies who live in one state but are born in another, and babies born in one hospital and then transferred to another. The medical home plays a key role in the care of infants who do not pass or do not receive a newborn hearing screening by helping families understand the EHDI process and encouraging prompt follow-up. The medical home can ensure that appropriate and timely steps are taken to identify children who are deaf and hard of hearing through rescreening and referrals for diagnostic evaluations and early intervention services (Mehl & Esquivel, 2016).
The following strategies have been identified to decrease loss to follow-up for infants who do not pass their hearing screening:
- Script the communication with parents/guardians regarding the screening result and the importance of follow-up.
- Standardize the process for collecting contact information at the time of the screening, including obtaining a second point of contact for the family and verifying the primary care physician or clinic responsible for follow-up.
- Schedule a follow-up appointment (rescreening or diagnostic) before the family leaves the hospital.
- Call the family to confirm the follow-up appointment and provide necessary assistance (e.g., transportation vouchers).
- Use efficient and effective forms and methods of communication between all parts of the care team (e.g., audiologist, physician) to share results and follow-up plans.
- Obtain consent from parents/guardians for release of information during the first contact with early intervention, so that information can be shared between early intervention providers, the physician, and the state EHDI database (Russ et al., 2010).
For more information, see Improving Follow-Up after Newborn Hearing Screening [PDF], an action kit for audiologists by the National Institute for Children’s Health Quality.
System Issues
The following tasks are ongoing in the effort to improve EHDI systems and reduce LTF and LTD rates in infants who do not pass their newborn hearing screening:
- Maintain consistent state and federal funding for EHDI programs.
- Increase the number of hospitals that involve audiologists in the newborn hearing screening program.
- Require hospital personnel to identify a newborn’s medical home and/or primary care physician (Health Resources and Services Administration [HRSA], 2009).
- Use designated physicians as AAP EHDI Chapter Champions to educate medical home providers and respond to issues regarding newborn hearing screening within the state (AAP, 2021).
- Increase parent/guardian education prior to screening, including the urgency of early diagnosis of hearing loss (Alexander & van Dyck, 2006).
- Coordinate and integrate data management among service providers.
- Encourage audiologists to report diagnostic results as recommended and required by state regulations.
Program Evaluation and Quality Assurance
The following quality indicators and benchmarks can be used to evaluate quality assurance and program performance relative to screening and diagnosis (JCIH, 2007):
- the percentage of newborns who complete hearing screening (inpatient and outpatient) by 1 month of age—benchmark of more than 95%
- the percentage of newborns referred for diagnostic audiologic evaluation—benchmark of less than 4%
- the percentage of newborns who did not pass the screening and went on to have a comprehensive diagnostic audiologic evaluation by 3 months of age—benchmark of 90%
- the percentage of infants obtaining amplification within 1 month of hearing loss confirmation for families choosing that option—benchmark of 95%
Other quality assurance indicators may include
- the number of follow-up appointments scheduled and recorded,
- parent/guardian satisfaction with the process,
- timeliness and accuracy of screening results, and
- the capacity to analyze and report data.
Evaluation of programs can include statewide findings reported to the CDC on the number of
- live births,
- newborns screened,
- missed screenings,
- newborns not screened due to parent/guardian refusal,
- newborns passing the screening prior to hospital discharge,
- newborns discharged not passing the screening in one or both ears,
- infants referred for diagnostic testing,
- infants who had inconclusive results,
- infants screened who were transferred in/out of a given facility,
- newborns passing outpatient (re)screening, and
- newborns not passing outpatient screening in one or both ears.
For more information, see the CDC resource on Early Hearing Detection and Intervention Information System (EHDI-IS) Functional Standards.
Laws and Regulations
Privacy regulations—including the Health Insurance Portability and Accountability Act, the Family Educational Rights and Privacy Act, Part C Privacy Regulations (Individuals with Disabilities Education Act), and state privacy reporting laws—may affect the sharing of information among service providers. Audiologists and other EHDI stakeholders must understand and abide by these regulations and develop procedures to ensure that information is shared in a timely manner to avoid loss to follow-up. See the National Center for Hearing Assessment and Management (2008) white paper, The Impact of Privacy Regulations [PDF]. Audiologists are responsible for understanding how privacy laws are interpreted in their specific states or programs.
All 50 states and the District of Columbia have EHDI programs established either by law or by voluntary compliance. Find contact information on the ASHA state-by-state resource under “Contact Information.”
Reimbursement and Program Funding
Newborn hearing screening funding varies from state to state. States procure funds from Medicaid, the Title V Maternal and Child Health (MCH) Block Grant program, state general revenues, and federal EHDI grant funding via the HRSA and the CDC . States often try to identify funding sources other than grants to ensure program continuity in case grant support becomes unavailable. Costs associated with newborn hearing screening include those associated with the tests (e.g., equipment, disposables, staff time) and those associated with program management (e.g., data entry, data analysis, follow-up activities, outcomes monitoring).
Early and Periodic Screening, Diagnostic and Treatment (EPSDT) Services
Through the EPSDT program, a set of services and benefits are mandated for all individuals under 21 years of age who are enrolled in Medicaid. Federal rules encourage partnerships between state Medicaid and Title V agencies to ensure better access to and receipt of the full range of screening, diagnostic, and treatment services. State Medicaid programs are required to cover EPSDT services offered to Medicaid-enrolled children.
The Early Hearing Detection and Intervention Act
In December 2022, Congress passed the Early Hearing Detection and Intervention Act (S.4052) reauthorizing the federal EHDI program for an additional 5 years. This program awards grant funding to state-based EHDI programs via the HRSA and the CDC. Every state or territory receives an equal share of HRSA grant funding, which is typically used to fund staff positions dedicated to the follow-up steps of the newborn hearing screening process (e.g., diagnostics, early intervention). The CDC awards grants competitively to a subset of states, with the aim of bolstering their data systems and tracking capabilities regarding successful connection to diagnostic and early intervention services. S. 4052 requires the Government Accountability Office to conduct a study, due in December 2024, assessing program performance and efforts to ensure that newborns, infants, and young children have access to timely hearing screenings and early interventions, particularly in medically underserved populations. The EHDI program will require reauthorization by Congress in 2027.
Current Procedural Terminology (CPT) Codes
There are several CPT codes used to report early hearing detection testing to a payer or program. The determination of which CPT codes are covered for screening and diagnostic testing is defined by the payer or program. For more information, see the ASHA resource on audiology coding as well as National Center for Hearing Assessment and Management State Contacts.
ASHA Resources
- ASHA State-by-State
- Audiology Coding Information by Topic
- Audiology Information Series: Newborn Hearing Screening [PDF]
- Early Hearing Detection and Intervention (EHDI)
- Health Literacy
- Hearing-Related Topics: Terminology Guidance
- Infection Control Resources for Audiologists and Speech-Language Pathologists
- Model Universal Newborn/Infant Hearing Screening, Tracking, and Intervention Bill
- Newborn Hearing Screening Protocol: NICU
- Newborn Hearing Screening Protocol: Well-baby Nursery
Other Resources
This list of resources is not exhaustive, and the inclusion of any specific resource does not imply endorsement from ASHA.
- American Academy of Audiology Position Statement on Early Identification of Cytomegalovirus in Newborns [PDF]
- American Academy of Pediatrics (AAP): Early Hearing Detection and Intervention
- AAP: Early Hearing Detection and Intervention: Resources
- Bright Futures/American Academy of Pediatrics: Recommendations for Preventive Pediatric Health Care [PDF]
- Centers for Disease Control and Prevention (CDC): Early Hearing Detection and Intervention Information System (EHDI-IS) Functional Standards
- CDC: Screening and Diagnosis of Hearing Loss
- Early Hearing Detection & Intervention: Pediatric Audiology Links to Services (EHDI-PALS)
- Health Resources & Services Administration: Title V Maternal and Child Health (MCH) Block Grant
- Joint Committee on Infant Hearing (JCIH)
- JCIH: Year 2019 Position Statement
- National Center for Hearing Assessment and Management (NCHAM)
- NCHAM: Medical Home
- NCHAM: Newborn Hearing Screening Training Curriculum
- NCHAM: Resources
- NCHAM: State Contacts
- NCHAM: The Impact of Privacy Regulations [PDF]
- National Institute for Children's Health Quality (NICHQ): Enhancing Communications: Improving Care for Infants with Hearing Loss [PDF]
- NICHQ: Improving Follow-Up after Newborn Hearing Screening: An Action Kit for Audiologists [PDF]
- S. 4052—Early Hearing Detection and Intervention Act of 2022
Alexander, D., & van Dyck, P. C. (2006). A vision of the future of newborn screening. Pediatrics, 117(Suppl. 3), S350–S354. https://doi.org/10.1542/peds.2005-2633O
American Academy of Pediatrics. (2021). Early hearing detection and intervention. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/PEHDIC/pages/early-hearing-detection-and-intervention.aspx
American Speech-Language-Hearing Association. (2016). Scope of practice in speech-language pathology [Scope of practice]. https://www.asha.org/policy/
American Speech-Language-Hearing Association. (2018). Scope of practice in audiology [Scope of practice]. https://www.asha.org/policy/
American Speech-Language-Hearing Association. (2023). Code of ethics [Ethics]. https://www.asha.org/policy/
Berg, A. L., Prieve, B. A., Serpanos, Y. C., & Wheaton, M. A. (2011). Hearing screening in a well-infant nursery: Profile of automated ABR-fail/OAE-pass. Pediatrics, 127(2), 269–275. https://doi.org/10.1542/peds.2010-0676
Berge, P. H. (1992). Setting limits on involuntary HIV antibody testing under Rule 35 and state independent medical examination statutes. Florida Law Review, 44(5), 767–805.
Centers for Disease Control and Prevention. (2023, January). 2020 summary of national CDC EHDI data. https://www.cdc.gov/ncbddd/hearingloss/2020-data/01-data-summary.html
Clayton, E. W. (1992). Issues in state newborn screening programs. Pediatrics, 90(4), 641–646. https://doi.org/10.1542/peds.90.4.641
D’Agostino, J. A., & Austin, L. (2004). Auditory neuropathy: A potentially under-recognized neonatal intensive care unit sequela. Advances in Neonatal Care, 4(6), 344–353. https://doi.org/10.1016/j.adnc.2004.09.007
Early Hearing Detection and Intervention Act of 2022, S. 4052, 117th Congress. (2021–2022). https://www.congress.gov/bill/117th-congress/senate-bill/4052
Fleischman, A. R., Post, L. F., & Dubler, N. N. (1994). Mandatory newborn screening for human immunodeficiency virus. Bulletin of the New York Academy of Medicine, 71(1), 4–17.
Harlor, A. D. B., Jr., & Bower, C. (2009). Hearing assessment in infants and children: Recommendations beyond neonatal screening. Pediatrics, 124(4), 1252–1263. https://doi.org/10.1542/peds.2009-1997
Health Insurance Portability and Accountability Act of 1996, Pub. L. No. 104-191, 110 Stat. 1938 (1996).
Health Resources and Services Administration. (2009). Enhancing communication: Improving care for infants with hearing loss. U.S. Department of Health and Human Services.
Johnson, J. L., White, K. R., Widen, J. E., Gravel, J. S., James, M., Kennalley, T., Maxon, A. B., Spivak, L., Sullivan-Mahoney, M., Vohr, B. R., Weirather, Y., & Holstrum, J. (2005). A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics, 116(3), 663–672. https://doi.org/10.1542/peds.2004-1688
Joint Committee on Infant Hearing. (2007). Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics, 120(4), 898–921. https://doi.org/10.1542/peds.2007-2333
Joint Committee on Infant Hearing. (2019). Year 2019 position statement: Principles and guidelines for early hearing detection and intervention programs. The Journal of Early Hearing Detection and Intervention, 4(2), 1–44.
Mehl, A., & Esquivel, M. (2016). Medical home & EHDI: The importance of appropriate & timely screening, diagnosis, management, & follow-up. In L. R. Schmeltz (Ed.), The NCHAM eBook: A resource guide for early hearing detection and intervention (pp. 10-1–10-5). National Center for Hearing Assessment and Management. https://www.ldh.la.gov/assets/oph/Center-PHCH/Center-PH/hearingspeechvision/EHDI_E_BOOK_10_Chapter10MedicalHome2014.pdf [PDF]
National Center for Hearing Assessment and Management. (2008). The impact of privacy regulations: How EHDI, Part C, & health providers can ensure that children & families get needed services [White paper]. https://www.infanthearing.org/privacy/docs/PrivacyWhitePaper.pdf [PDF]
Norton, S. J., Gorga, M. P., Widen, J. E., Folsom, R. C., Sininger, Y., Cone-Wesson, B., Vohr, B. R., Mascher, K., & Fletcher, K. (2000). Identification of neonatal hearing impairment: Evaluation of transient evoked otoacoustic emission, distortion product otoacoustic emission, and auditory brain stem response test performance. Ear and Hearing, 21(5), 508–528.
Russ, S. A., Hanna, D., DesGeorges, J., & Forsman, I. (2010). Improving follow-up to newborn hearing screening: A learning-collaborative experience. Pediatrics, 126(Suppl. 1), S59–S69. https://doi.org/10.1542/peds.2010-0354K
Siegel, J. D., Rhinehart, E., Jackson, M., Chiarello, L., & the Healthcare Infection Control Practices Advisory Committee. (2007). 2007 guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings. https://www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf [PDF]
Turchetta, R., Orlando, M. P., Cammeresi, M. G., Altissimi, G., Celani, T., Mazzei, F., Giacomello, P., & Cianfrone, G. (2012). Modifications of auditory brainstem responses (ABR): Observations in full-term and pre-term newborns. The Journal of Maternal-Fetal & Neonatal Medicine, 25(8), 1342–1347. https://doi.org/10.3109/14767058.2011.634457
Vohr, B. R., Oh, W., Stewart, E. J., Bentkover, J. D., Gabbard, S., Lemons, J., Papile, L.-A., & Pye, R. (2001). Comparison of costs and referral rates of 3 universal newborn hearing screening protocols. The Journal of Pediatrics, 139(2), 238–244. https://doi.org/10.1067/mpd.2001.115971
Yoshinaga-Itano, C., DeConde Johnson, C., Carpenter, K., & Stredler Brown, A. (2008). Outcomes of children with mild bilateral hearing loss and unilateral hearing loss. Seminars in Hearing, 29(2), 196–211. https://doi.org/10.1055/s-2008-1075826
Acknowledgments
Content for ASHA’s Practice Portal is developed through a comprehensive process that includes multiple rounds of subject matter expert input and review. ASHA extends its gratitude to the following subject matter experts who were involved in the development of the Newborn Hearing Screening page:
- Kathryn Beauchaine, MA, CCC-A
- Tara Carroll, MCD, CCC-A
- Brandt Culpepper, PhD, CCC-A
- Janet Farrell
- Jeffrey Hoffman, PhD, CCC-A
- Michelle King, AuD, CCC-A
- Patti Martin, PhD, CCC-A
- Ryan McCreery, PhD
- Mary Pat Moeller, PhD, CCC-A
- Beth Prieve, PhD, CCC-A
- Jackson Roush, PhD, CCC-A
- Diane Sabo, PhD, CCC-A
- Lynn Spivak, PhD, CCC-A
- Anne Marie Tharpe, PhD, CCC-A
- Randi Winston-Gerson, AuD, CCC-A
In addition, ASHA thanks the members of the Newborn Hearing Screening Working Group whose work was foundational to the development of this content. Members of the working group included Beth Prieve (chair), Kathryn Beauchaine, Diane Sabo, Anne Marie Tharpe, and Anne Oyler (ex officio). ASHA Vice Presidents for Professional Practices in Audiology Jaynee Handelsman (2010–2012) and Donna Fisher Smiley (2013–2015) served as the monitoring officers.
ASHA also thanks the members of the Working Group on Loss to Follow-Up whose work was foundational to the development of this content. Members of the working group included Anne Marie Tharpe (chair), John Eichwald, Janet Farrell, Jeffrey Hoffman, Meredith Isola, Patti Martin, Amy M. Robbins, Lynn Spivak, Susan Wiley, and Pam Mason (ex officio). ASHA Vice President for Professional Practice in Audiology Gwendolyn D. Wilson (2007–2009) served as the monitoring officer.
Citing Practice Portal Pages
The recommended citation for this Practice Portal page is:
American Speech-Language-Hearing Association. (n.d.). Newborn hearing screening [Practice portal]. https://www.asha.org/Practice-Portal/Professional-Issues/Newborn-Hearing-Screening/
Content Disclaimer: The Practice Portal, ASHA policy documents, and guidelines contain information for use in all settings; however, members must consider all applicable local, state and federal requirements when applying the information in their specific work setting.